A process thought to protect the kidneys may trigger rapid cyst growth in people with polycystic kidney disease, a new study shows.
For people with polycystic kidney disease (PKD), life can be a never-ending cycle of symptoms: aches and pains, abdominal swelling, kidney stones, and high blood pressure. The disease frequently leads, at worst, to a suite of major issues, including kidney failure, cysts in the liver, and vascular problems, including strokes.
PKD is a “fairly common genetic disorder,” according to the National Institutes of Health. It affects roughly 600,000 people in the United States, with the more common autosomal dominant (AD) form affecting roughly one in 500 to 1,000 people.
The disease remains somewhat of a mystery and has no cure, researchers say. Meanwhile, treatment of various symptoms and complications put a heavy economic burden on the healthcare system, and dramatically lower patients’ quality of life.
“Most patients will eventually form these big cystic kidneys, and they will need dialysis or a kidney transplant, both of which are not great options,” says Thomas Weimbs, a biochemist at the University California, Santa Barbara.
‘Third-hit’ trigger
In a step toward disrupting the cycle that leads to cyst formation in the kidneys, the Weimbs lab uncovered a previously unrecognized mechanism that accelerates cystogenesis. The rapid dilation of the tubules that conduct waste away from the kidneys in the form of urine is a “third-hit” trigger that results in rapid cyst growth, according to the paper in the Journal of Clinical Investigation.
The kidneys are the hard-working filtration system that removes waste from the blood. Blood enters the nephrons (the kidneys’ basic functional unit) where waste and fluid pass through the renal tubules, while cells and proteins remain in the blood. Some fluid and nutrients get reabsorbed into circulation while excess fluid and waste become urine that flows to the bladder. Each human kidney contains roughly a million such tubules, Weimbs says.
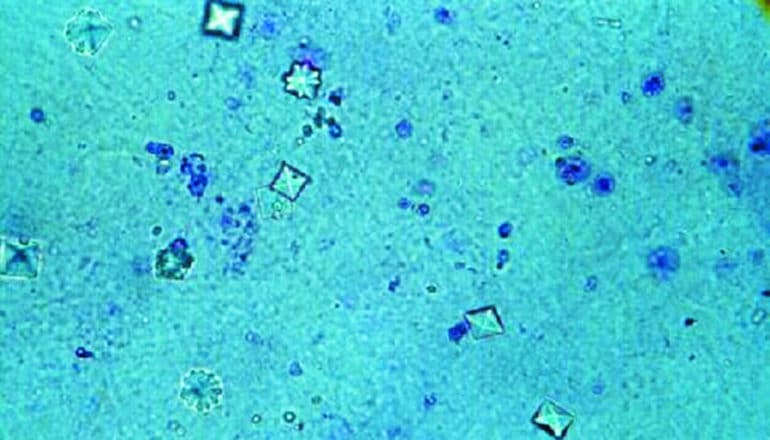
During this filtration process, waste products—such as calcium oxalate, calcium phosphate, and uric acid—tend to concentrate and precipitate into crystals in the renal tubules. In healthy people, these millions of microscopic crystals form but flush away with the urine, while other factors prevent the runaway growth and retention of these crystals in the tubules. The formation and accumulation of these crystals, if left unchecked, can lead to kidney stones.
To prepare to flush out these crystals the renal tubules rapidly dilate, and then return to normal after the crystals have cleared. This dilation is a mechanism that scientists had not previously recognized, Weimbs says.
“It was not understood how the bulk of these crystals are flushed out,” he says. Until now, researchers thought stuck crystals crossed through into the kidneys’ interstitial tissue to be reabsorbed, but the new research shows that’s not the case for most crystals.
Crystals and cysts
In normal-functioning kidneys, the tubule dilation acts as a protective mechanism. The deposition of oxalate crystals in particular triggers the rapid activation of protein signaling pathways (mTOR and Src/STAT3) that regulate cell growth and proliferation, accompanied by the rapid dilation of the entire tubule system to dislodge the microcrystals.
“In kidneys genetically preconditioned to form these cysts, we found that these crystals can trigger the same dilation, but instead of going back to normal those tubules overshoot and form cysts,” Weimbs says.
In people with ADPKD, the rapid and constant tubule dilation is seen as a “third hit” physical injury that results in cyst formation. According to the “third hit” model of cystogenesis, three events must occur to form individual cysts: the first two are genetic mutations, while the third is a physiological damage/repair response, resulting in an overcompensation by the renal tubule that leads to formation of the fluid-filled sacs.
Trauma and other assaults to the kidneys are fairly rare, Weimbs says, but the microcrystals could present a persistent and relevant type of injury in ADPKD patients that could trigger the damage/repair response.
The findings suggest that contrary to conventional assumptions that abnormalities in tissue architecture or metabolic abnormalities during ADPKD progression lead to increased kidney stones, the opposite may be the case: More crystals lead to the progression of ADPKD. Additionally, it is possible that ADPKD progression and kidney stone formation reinforce each other, the study shows.
This opens up the possibility that the same well-established practices for keeping kidney stones at bay may also prove effective for slowing the progression of ADPKD, Weimbs says. “Our research suggests that the rate of progression could be at least in part determined by something like diet.”
Recommendations for preventing kidney stones, such as avoiding certain foods, increasing water intake, and prescription citrate therapy, could also prove beneficial for those with polycystic kidney disease, he says.
Additional collaborators on the work are from UC Santa Barbara; the Mayo Clinic College of Medicine; University Children’s Hospital Bonn in Germany; the University of Oklahoma; the University of Messina in Italy; the University of Florida; and the University of Alabama.
Source: UC Santa Barbara
The post How crystals trigger cyst growth in serious kidney disease appeared first on Futurity.


No comments:
Post a Comment